Acid-base balance (sometimes called acid-alkaline balance) can be a complex and confusing topic. Today, I want to simplify it and give you the essential info you need to look after and improve your health. So stick with me and I’ll take you through it from a naturopath’s perspective.
Even if chemistry has never been your thing you might remember back to school days and the lesson on pH. There was talk of “acids”, “bases” and “buffers”. You might recall litmus paper that turned pink or blue when dipped in different substances depending on that substance’s pH. The numbers for pH range from 1 (being very acidic) to 14 being very alkaline (or base) with a pH of 7 being neutral.
Your blood has a normal pH of 7.35-7.45 (just slightly more alkaline than the neutral point of 7). It’s a pretty small range and any variance outside this range has significant detrimental consequences for your health, potentially a medical emergency. So, as you can imagine, your body has some pretty neat ways of ensuring you maintain that critical number at all times. These systems are your buffering systems.
The problem is, every food and substance you put in your mouth has the potential to impact your pH, pushing it one way or the other (remember the litmus paper). Furthermore, your own essential metabolic processes result in end-products that also have an impact on your blood pH.
Don’t confuse acid taste with acid pH*
It’s easy to confuse the concept of acid-alkaline balance with foods that taste “acidic” (e.g. lemons). But what we’re talking about is how that food is metabolised or broken down, and the effect the end substance has on your blood pH. To take the lemon as an example, it tastes acidic or tart but when digested its effect on your blood is actually alkaline.
We measure the impact of food on your blood pH by something called the Potential Renal Acid Load (PRAL). In simple terms, PRAL is a measure of the net acidic effects of different foods once metabolised (and therefore a measure of how hard your body needs to work to buffer that acid load).
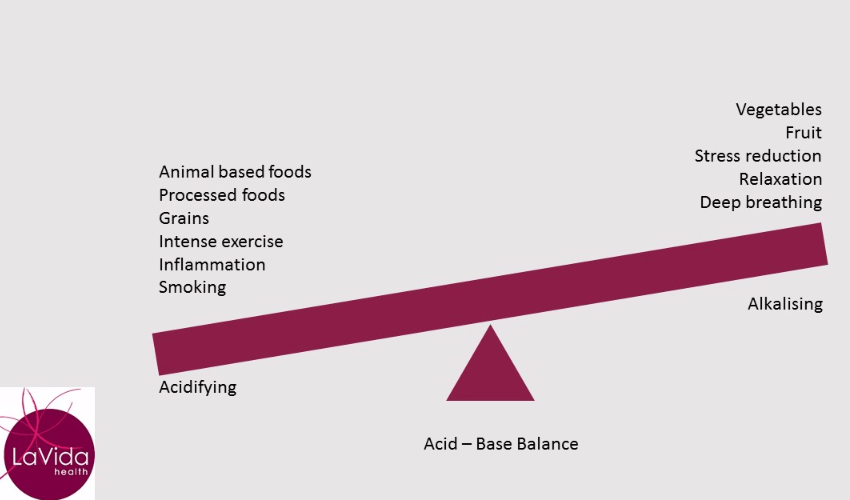
In general, fruit and vegetables have a lower PRAL and therefore an alkalising effect on your body. Animal foods (including fish, dairy and eggs), sugar and grains have a higher PRAL and therefore put more acid into your system (which then requires buffering).
As an example, you would need to eat 300g of vegetables to offset the acid load of 100g of meat.
Why do you need to consider your acid-base balance?
If I have these buffering systems to help keep my blood pH in the safe range why do I need to worry if my diet has a higher acid load? Good question!
Do you eat a fairly typical Australian diet consisting of meat, dairy, eggs, processed foods, lots of grain foods and sugar but low in fruit and vegetables?
If so, you will be placing constant demands on your buffering systems. If you smoke, take drugs or drink a large amount of alcohol you further burden your system. Add to this the metabolic by-products of your own biochemistry. These include the effects of stress, intense exercise, inflammation, disease and aging which all have a net acid load on your body.
As a consequence, your buffering systems will be working very hard to maintain that slightly alkaline blood pH.
To maintain your blood pH, you will draw on your supplies of alkalising minerals such as calcium, magnesium, potassium and sodium. These come from your tissues, especially your bones and muscles. You also excrete acid via the kidneys (so remember to drink water), however, your ability to do this declines by approximately 1% per year from the age of thirty.
Eventually, your buffering systems can’t keep up with the acid burden and you end up in a state of chronic mild metabolic acidosis.
The possible signs of acid-base imbalance
Mild metabolic acidosis can go unnoticed for years however there are some clues that I look out for that could indicate a problem with your acid-base buffering systems. These include:
- Headaches
- Generalised weakness or unusual muscle fatigue
- Loss of appetite
- Nausea and vomiting or just feeling “off colour”
- Confusion
- Anxiety
Conditions which can be exacerbated or caused by prolonged mild metabolic acidosis include:
- Kidney disease and kidney stones
- Osteoporosis or osteopenia
- Loss of muscle (known as sarcopenia)
- Insulin resistance and high blood glucose
- Fatigue
- High blood pressure
- Inflammation and pain
- Gout
- Difficulty losing weight
- Impaired thyroid function
Do any of these apply to you?
Obviously, there might be other reasons behind these conditions as well, but addressing acid-base balance should not be overlooked.
As a naturopath how do I assess your acid-base picture?
There are a number of aspects to consider when taking your case history. Obviously your diet and lifestyle will be explored in detail. I will also look for signs of magnesium or calcium deficiency. A high demand for these nutrients can be a clue to an acid-base imbalance.
Another measure can be determined from your general pathology tests when your blood electrolytes are assessed. From your results we can look at your blood bicarbonate levels as well as work out your anion gap. This is a clue as to how hard your buffering systems are working to maintain blood pH.
A further test can measure your urinary pH. Your urine is one of the end products of your metabolism so an acidic urinary pH can be a clue to what’s happening in your body.
There are other, more sophisticated tests as well but the above assessments are a good place to start.
Where to from here?
The first thing to do is to consider how much of an acid load you are placing on your body. Think about the following. How many of them apply to you?
- a diet low in fruit and vegetables and high in animal foods, grains and processed foods
- stress
- smoking or alcohol
- regular intensive exercise
- pain or inflammation somewhere in your body
- a diagnosis of osteopenia or osteoporosis
- weight gain or blood sugar issues
- history of kidney stones or gout
- taking certain pharmaceutical medications on a regular basis
Rest assured that if you are a naturopathic client of mine, we will be watchful for the possibility of an acid-base imbalance and will implement appropriate dietary and nutritional treatments to reduce your acid burden.
If you think your acid-base balance could be contributing to how lousy you feel, book a free 15-minute Clarity Call (below) and let’s have a chat about it.
*As a side note, don’t confuse acid in the context of blood pH with “stomach acid”. The acid in your stomach (which is essential for your digestion) has a pH of around 1.5-3.5. It is highly acidic as a first line of defence against any bugs you might pick up from the food you eat and helps break food down into nutrients you can use.




4 thoughts on “What you need to know about acid-base balance for good health”
My first introduction to acid and base was in the primary classes, but I never assumed that they make such an effect on our health too..!! Thanks for clearing so many doubts related to our body acids and the vital role they play in our health.
Thanks Celine. Supporting your own buffering systems through your diet can make a big difference to how you feel.
Thank you Kaye for more interesting and useful information.
Glad you found it useful, Jane.